Navigating the complexities of regulatory submissions can be a daunting task. Fortunately, Ectd document templates offer a lifeline, simplifying the process and ensuring compliance. These pre-formatted templates provide a structured framework, guiding users through the creation of high-quality submissions that meet the stringent requirements of regulatory authorities.
By leveraging Ectd document templates, pharmaceutical companies can save time, reduce errors, and enhance the overall efficiency of their regulatory submissions. Let’s delve into the world of Ectd document templates, exploring their benefits, best practices, and how they can revolutionize your regulatory strategy.
Ectd Document Templates: An Overview

Intro Paragraph
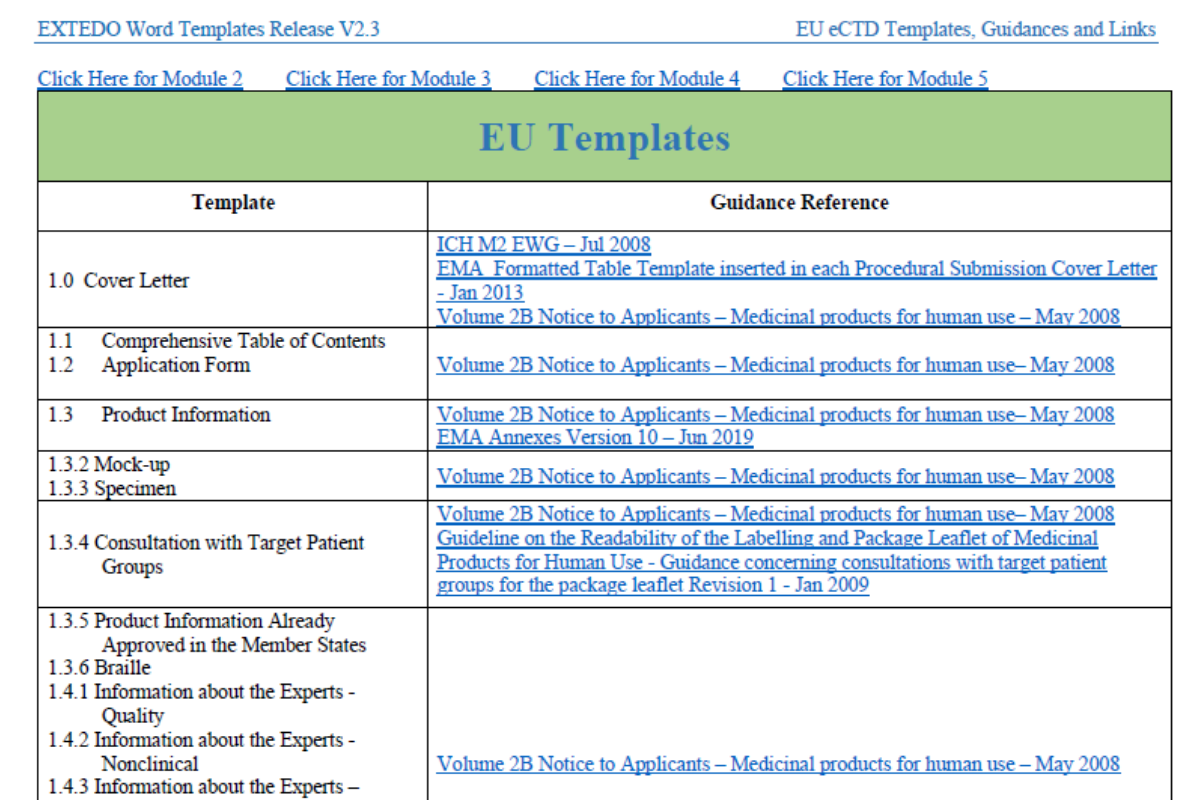
Ectd document templates are pre-defined, standardized templates used in the electronic submission of regulatory documentation to regulatory agencies. These templates ensure consistency, accuracy, and completeness of submissions, making the review process more efficient and effective.
Explanatory Paragraph
Using Ectd document templates offers several benefits, including:
– Time savings: Templates eliminate the need to create documents from scratch, saving time and effort.
– Consistency: Standardized templates ensure that all submissions adhere to the same format and structure, facilitating easy comparison and review.
– Accuracy: Pre-defined templates minimize errors and omissions, enhancing the quality of submissions.
– Compliance: Templates align with regulatory requirements, ensuring compliance and reducing the risk of rejections.
Common Ectd Document Templates
Common Ectd document templates include:
– Module 1: Administrative Information
– Module 2: Common Technical Document
– Module 3: Quality
– Module 4: Non-Clinical Study Reports
– Module 5: Clinical Study Reports
Importance of Standardized Templates
Standardized templates are crucial for Ectd submissions because they:
– Facilitate automated processing and review by regulatory agencies.
– Enable efficient data exchange and collaboration among stakeholders.
– Ensure that submissions meet the required standards and specifications.
– Promote transparency and consistency in regulatory submissions.
Creating Ectd Document Templates

Wagwan, fam! Creating Ectd document templates is like making a sick mixtape that’s gonna drop your socks off. Here’s a guide to help you craft templates that’ll make your life lit.
First off, keep it simple, innit? Don’t go overboard with fancy designs or jargon that’ll make your templates a headache to use. Stick to clear and concise language that everyone can understand.
Tips for Designing Effective Ectd Document Templates
- Use a consistent layout and structure throughout all your templates.
- Include clear headings and subheadings to make it easy to navigate.
- Use bullet points and tables to present information in a visually appealing way.
- Proofread your templates carefully before using them to avoid any embarrassing typos.
Best Practices for Creating Ectd Document Templates
- Start with a template that’s already been created. This will save you a lot of time and effort.
- Customize the template to meet your specific needs.
- Share your templates with other members of your team.
- Update your templates regularly to keep them current.
Ectd Document Templates for Specific Modules
Organising Ectd document templates by modules (e.g., Module 1, Module 2, Module 3) helps in streamlining the document management process. This allows for easy access and retrieval of specific templates when needed.
Module-wise Ectd Document Templates
The table below lists Ectd document templates for each module, along with links to download or access them:
| Module | Ectd Document Templates | Download/Access Link |
|—|—|—|
| Module 1 | Administrative Information Template | [Link to Download] |
| Module 1 | Common Technical Document Template | [Link to Download] |
| Module 2 | Quality Overall Summary Template | [Link to Download] |
| Module 2 | Non-Clinical Overview Template | [Link to Download] |
| Module 3 | Clinical Study Report Template | [Link to Download] |
| Module 3 | Clinical Overview Template | [Link to Download] |
Ectd Document Templates for Different Regulatory Authorities
Ectd document templates are essential for ensuring the efficient and accurate submission of regulatory documentation to different regulatory authorities around the world. Different regulatory authorities have specific requirements for the format and content of Ectd submissions, making it crucial to use templates that meet these requirements.
The table below lists some of the key regulatory authorities and provides information on their specific Ectd document template requirements:
Table: Ectd Document Templates for Different Regulatory Authorities
| Regulatory Authority | Ectd Document Template Requirements |
|---|---|
| FDA (United States) |
|
| EMA (European Union) |
|
| PMDA (Japan) |
|
It is important to use Ectd document templates that meet the requirements of the target regulatory authority. Using the correct templates will help to ensure that your submission is complete, accurate, and compliant with the relevant regulations.
Advanced Ectd Document Templates
Advanced Ectd document templates are the next level of efficiency and accuracy for your Ectd submissions. They offer a range of sophisticated features that can help you streamline your workflow, improve data quality, and ensure compliance.
In this section, we’ll explore the advanced features of Ectd document templates, demonstrate how to use them to enhance your submissions, and provide examples of advanced Ectd document templates that you can use to get started.
Conditional Formatting
Conditional formatting allows you to apply different formatting styles to different sections of your document based on specific criteria. For example, you could highlight all sections that require additional information in red or bold all sections that have been approved by a regulatory authority.
Conditional formatting can be a powerful tool for quickly identifying and addressing issues in your Ectd submissions.
Macros
Macros are small programs that can be used to automate repetitive tasks. In Ectd document templates, macros can be used to perform a variety of tasks, such as:
- Inserting standard text or images
- Populating fields with data from a database
- Generating reports
Macros can save you a lot of time and effort, and they can also help to ensure that your Ectd submissions are consistent and accurate.
Validation
Validation checks ensure that the data in your Ectd submission is accurate and complete. Ectd document templates can be configured to perform a variety of validation checks, such as:
- Checking that all required fields are filled in
- Checking that data is in the correct format
- Checking that data is within a specified range
Validation checks can help you to catch errors early in the submission process, which can save you time and effort in the long run.
Example Advanced Ectd Document Templates
There are a number of advanced Ectd document templates available online. Some of the most popular templates include:
- The EudraCT template, which is used for clinical trial applications in the European Union
- The FDA’s IND template, which is used for Investigational New Drug applications in the United States
- The PMDA’s CTD template, which is used for marketing authorization applications in Japan
These templates are a great starting point for creating your own advanced Ectd document templates.
Answers to Common Questions
What are the key benefits of using Ectd document templates?
Ectd document templates offer numerous benefits, including reduced submission time, improved data quality, enhanced compliance, and increased efficiency throughout the regulatory submission process.
How do I create effective Ectd document templates?
Effective Ectd document templates should be well-organized, easy to navigate, and compliant with regulatory requirements. Consider using industry-standard tools, following best practices, and seeking guidance from experts to ensure the highest quality templates.
Where can I find Ectd document templates for different regulatory authorities?
Ectd document templates tailored to specific regulatory authorities, such as the FDA, EMA, and PMDA, are available from various sources, including regulatory websites, industry associations, and third-party providers.
How can I use advanced features of Ectd document templates to enhance my submissions?
Advanced features of Ectd document templates, such as automated data validation, dynamic content insertion, and electronic signatures, can significantly enhance the quality and efficiency of regulatory submissions.